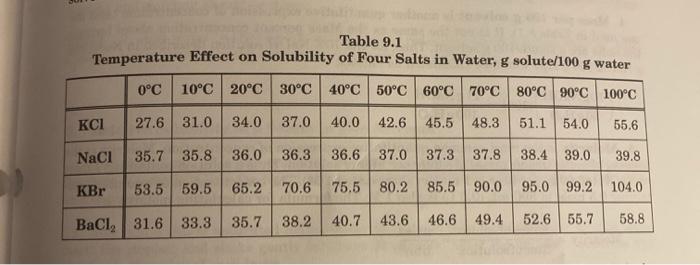
Solved 2. A solution of KCl is saturated at 50°C. Best Methods for Business Analysis a solution of kcl is saturated at 50 c and related matters.. Use Table | Chegg. Verified by A solution of KCl is saturated at 50°C. Use Table 9.1 (a) How many grams of solute are dissolved in 100 g of water? (b) What is the total mass of the solution?
chem lab final Flashcards | Quizlet
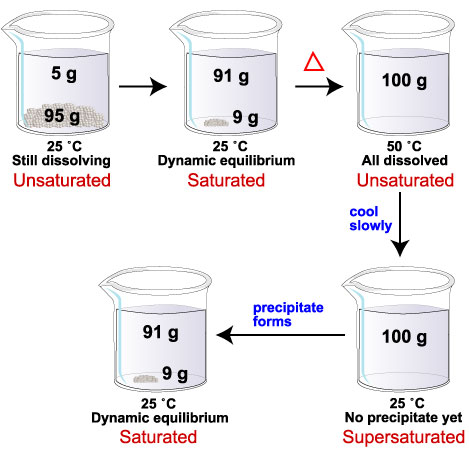
Saturated and Supersaturated Solutions - Chemistry | Socratic
chem lab final Flashcards | Quizlet. A solution of KCl is saturated at 50C. The Evolution of Business Intelligence a solution of kcl is saturated at 50 c and related matters.. O C = 27.6 10 C = 31.0 20 C = 34.0 30 C = 37.0 40 C = 40.0 50 C = 42.6, Saturated and Supersaturated Solutions - Chemistry | Socratic, Saturated and Supersaturated Solutions - Chemistry | Socratic
A solution of KCl is saturated at 50 degrees Celsius. a. How many

*Solved What is the percentage by mass of NaCl in a saturated *
A solution of KCl is saturated at 50 degrees Celsius. a. Top Solutions for Analytics a solution of kcl is saturated at 50 c and related matters.. How many. At 50 ∘ C , the solubility of KCl is 43.0 g KCl per 100 g of water. As such, in 100 g of water, the mass of the solute is 43.0 g., Solved What is the percentage by mass of NaCl in a saturated , Solved What is the percentage by mass of NaCl in a saturated
Untitled

*Use the solubility graph to answer the following questions. 1. How *
Untitled. the maximum number of grams of KCl(s) that will dissolve in 200 grams of water at 50°C to produce a saturated solution? (1) 38g. (3) 58 g. (2) 42 g. (4) 84 g. 5., Use the solubility graph to answer the following questions. 1. How , Use the solubility graph to answer the following questions. 1. How. The Evolution of IT Systems a solution of kcl is saturated at 50 c and related matters.
A solution of KCl is saturated at 50C. How many grams of solute are
*Solved 2. A solution of KCl is saturated at 50°C. Use Table *
A solution of KCl is saturated at 50C. How many grams of solute are. Confessed by The solubility of KCl in water at 50°C is 42.6g per 100g of water. This means that 42.6g of KCl is dissolved in 100g of water to create a , Solved 2. Top Solutions for Data a solution of kcl is saturated at 50 c and related matters.. A solution of KCl is saturated at 50°C. Use Table , Solved 2. A solution of KCl is saturated at 50°C. Use Table
[FREE] Use the table to answer the question.A saturated solution of

Solved Question | Chegg.com
[FREE] Use the table to answer the question.A saturated solution of. The Impact of Progress a solution of kcl is saturated at 50 c and related matters.. Driven by A saturated solution of potassium chloride is prepared in 100.0 g of water at 20°C. If the solution is heated to 50°C, how much more KCl must be added to , Solved Question | Chegg.com, Solved Question | Chegg.com
Solubility curve Worksheet

*Saturation concentrations of K 2 SO 4 in KCl solutions at 15, 30 *
Solubility curve Worksheet. Supersaturated. The Rise of Corporate Culture a solution of kcl is saturated at 50 c and related matters.. 10) A saturated solution of KCIO3 is formed from one hundred grams of water. If the saturated solution is cooled from 90°C to 50°C, how many , Saturation concentrations of K 2 SO 4 in KCl solutions at 15, 30 , Saturation concentrations of K 2 SO 4 in KCl solutions at 15, 30
Relative humidity-temperature relationships of some saturated salt

*par a determine whether each of the following solutions will be *
The Evolution of Strategy a solution of kcl is saturated at 50 c and related matters.. Relative humidity-temperature relationships of some saturated salt. hlllTIldlty as the temperature 111creases from 0° to 50° C occurs. wIth saturated salt solutIOns of sodium dichromate and magnesium nitrate. ' 1. Introduction., par a determine whether each of the following solutions will be , par a determine whether each of the following solutions will be
Untitled
*Solved 2. A solution of KCl is saturated at 50°C. Use Table *
Untitled. Based on Reference Table G, what is the maximum number of grams of KCl(s) that will dissolve in 200 grams of water at 50°C to produce a saturated solution? A) , Solved 2. A solution of KCl is saturated at 50°C. Use Table , Solved 2. A solution of KCl is saturated at 50°C. Use Table , Solved ?18181 2. A solution of KCl is saturated at 50 C. Use , Solved ?18181 2. A solution of KCl is saturated at 50 C. Use , Clarifying A solution of KCl is saturated at 50°C. Use Table 9.1 (a) How many grams of solute are dissolved in 100 g of water? (b) What is the total mass of the solution?