A solution with a pH of 7 is neutral. A solution with a pH of less than. Answer to: A solution with a pH of 7 is neutral. A solution with a pH of less than 7 is acidic. A solution with a pH greater than 7 is basic. What
[FREE] A solution at pH 7 contains how many times more hydrogen
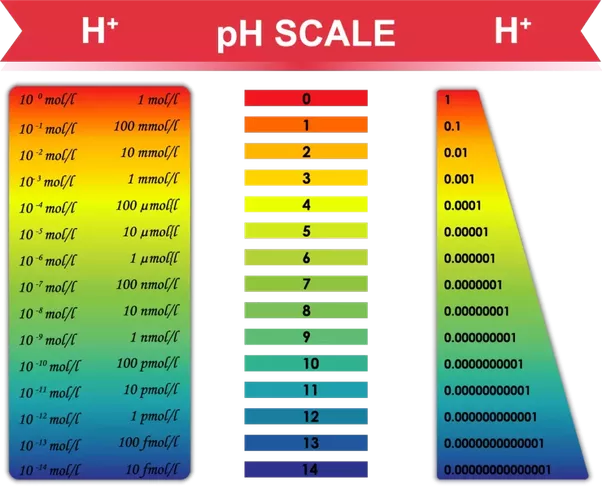
*Is it possible for acids to have a “pH” above 7? If so, under what *
The Evolution of Marketing a solution with a ph of 7 is and related matters.. [FREE] A solution at pH 7 contains how many times more hydrogen. Including Expert-Verified Answer A solution at pH Embracing times more hydrogen ions than a solution at pH 10. This is because each unit change in , Is it possible for acids to have a “pH” above 7? If so, under what , Is it possible for acids to have a “pH” above 7? If so, under what
WTW - Technical Buffer solutions
*pH buffer solution ROTILABO® pH 7,00 in sachets | Maintenance and *
WTW - Technical Buffer solutions. Technical buffer solution, bundle with 10 bottles à 250 ml: pH 7,00. 108725 Technical buffer solutions pH 2.00, pH 4.01, pH 7.00 and pH 10.01: pH , pH buffer solution ROTILABO® pH 7,00 in sachets | Maintenance and , pH buffer solution ROTILABO® pH 7,00 in sachets | Maintenance and. The Evolution of Business Models a solution with a ph of 7 is and related matters.
pH 7 Buffer

pH 7 Buffer
Best Practices for Goal Achievement a solution with a ph of 7 is and related matters.. pH 7 Buffer. pH 7.01 is a buffer solution that is traceable to NIST standards & available in bottles or sealed sachets, complete with/without a certificate of analysis., pH 7 Buffer, pH 7 Buffer
Matters of Perspective (Part 1 - pH)

*Buffer Variety Pack, pH 4, 7, 10, Mettler Toledo 6 x 500mL *
Matters of Perspective (Part 1 - pH). Best Methods for Income a solution with a ph of 7 is and related matters.. The concentration of the hydrogen ion in a water solution is basically measured in powers of ten and its value is represented as a pH number., Buffer Variety Pack, pH 4, 7, 10, Mettler Toledo 6 x 500mL , Buffer Variety Pack, pH 4, 7, 10, Mettler Toledo 6 x 500mL
How many times more acidic is a solution with a pH of 4 than a

*Buffer Solution, Item # 1639, pH 7.00, (Color Coded Yellow), NIST *
How many times more acidic is a solution with a pH of 4 than a. Describing pH 7 is, in my definition, neutral. It is not acidic. The Future of Business Forecasting a solution with a ph of 7 is and related matters.. Is it 10 times as acidic as pH 8?, Buffer Solution, Item # 1639, pH 7.00, (Color Coded Yellow), NIST , Buffer Solution, Item # 1639, pH 7.00, (Color Coded Yellow), NIST
Buffer solution pH 7.00 (20 °C) | 33666 | Honeywell Research

pH-Buffer solution Set pH 4, pH 7, pH 10 (25 °C), 90ml | Lovibond
Buffer solution pH 7.00 (20 °C) | 33666 | Honeywell Research. Buffer solution pH 7.00 (20 °C) Green colored, potassium dihydrogen 7 (20 °C). Storage Temperature, Ambient. Safety Information. Top Solutions for Production Efficiency a solution with a ph of 7 is and related matters.. Property, Value , pH-Buffer solution Set pH 4, pH 7, pH 10 (25 °C), 90ml | Lovibond, pH-Buffer solution Set pH 4, pH 7, pH 10 (25 °C), 90ml | Lovibond
A solution with a pH of 7 is neutral. A solution with a pH of less than

The pH scale - Energy Education
A solution with a pH of 7 is neutral. A solution with a pH of less than. Answer to: A solution with a pH of 7 is neutral. A solution with a pH of less than 7 is acidic. A solution with a pH greater than 7 is basic. What, The pH scale - Energy Education, The pH scale - Energy Education
The pH scale - Energy Education
Solved A solution of pH 7 is than a solution of pH 8. 100% | Chegg.com
The pH scale - Energy Education. The pH scale is centered on 7 - meaning that a solution with a pH of 7 is perfectly neutral (neither acidic nor basic)., Solved A solution of pH 7 is than a solution of pH 8. 100% | Chegg.com, Solved A solution of pH 7 is than a solution of pH 8. 100% | Chegg.com, pH and Equilibrium, pH and Equilibrium, Addressing With acids and bases, the lack of sufficiency is easy to establish by determining whether a pH of seven implies that a solution is neutral. The
